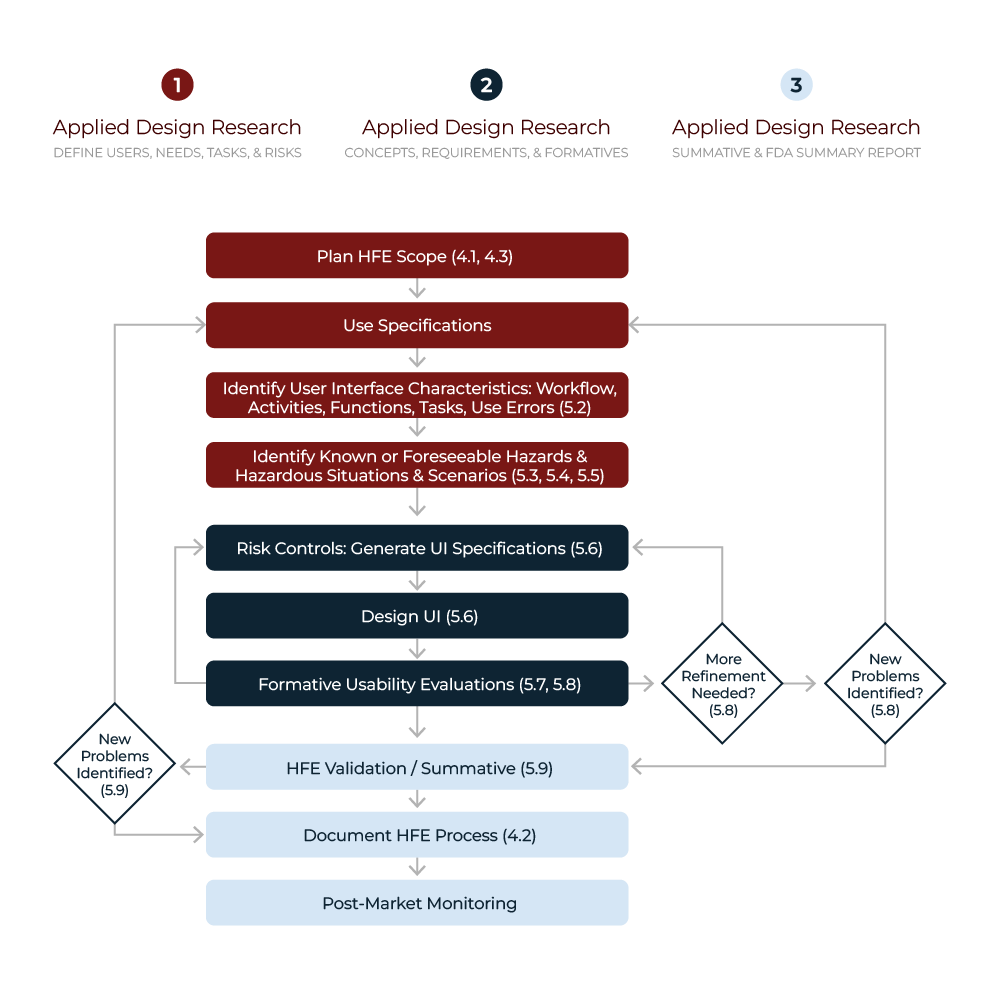
A Usability Engineering File (UEF) actively compiles a comprehensive set of documents that record every aspect of the usability engineering process for medical devices. It thoroughly details user research, risk assessments, usability evaluations, and design validation activities. The UEF not only provides essential evidence of regulatory compliance but also ensures the device consistently meets usability standards and requirements.
At Human Factors Design Consulting, we can thoroughly assess your product’s current design and documentation for UEF compliance. If any components are missing to meet FDA and 62366-1 requirements, we will promptly identify them. Moreover, we offer a clear roadmap to help you complete any remaining requirements.