What is Summative Evaluation / Human Factors Validation?
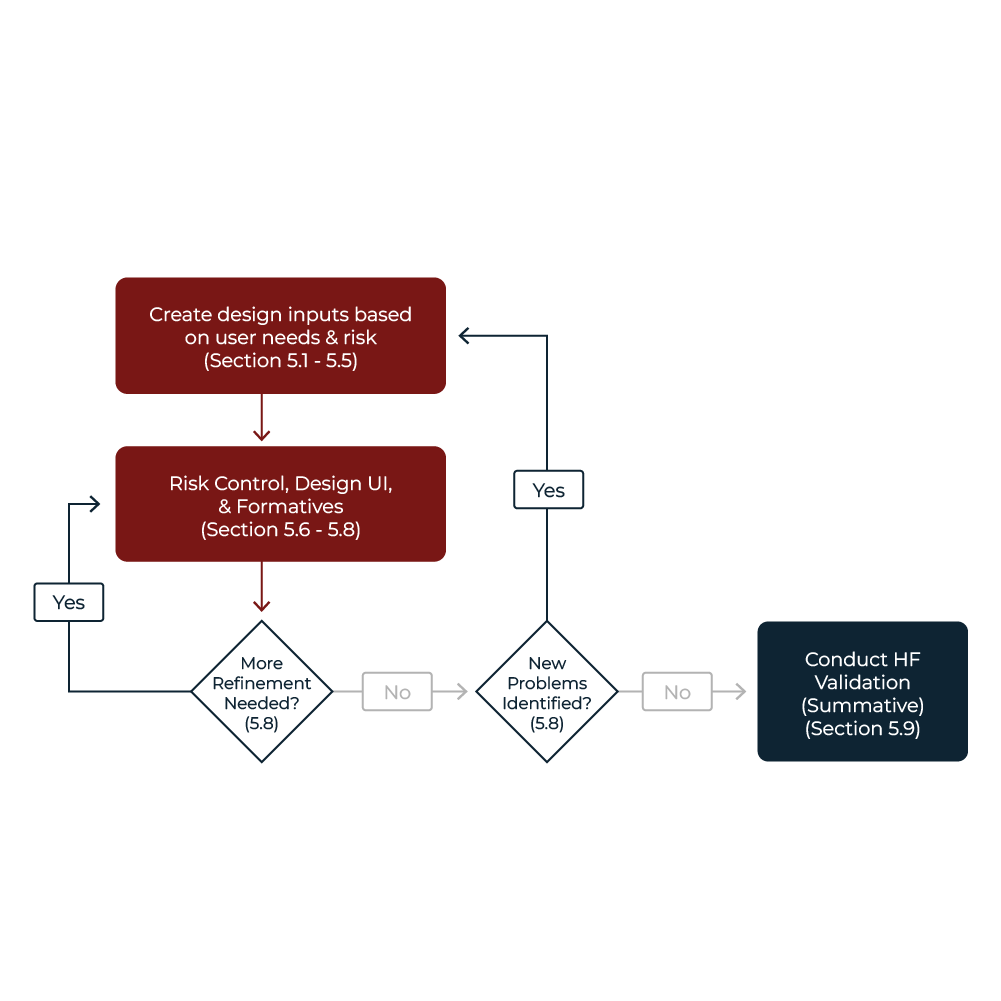
Summative Human Factors (HF) Validation Testing plays a critical role in the final stages of medical products development. Conducted during the validation phase of design controls, this testing assesses how users interact with the final or equivalent device user interface (UI) to identify potential use errors that could harm patients or users.
In this process, participants from the intended user population use the product in realistic simulated-use or actual-use scenarios, evaluating all critical tasks.
Summative validation aims to provide conclusive evidence that the medical device is safe and effective, ensuring it meets regulatory requirements before market release.
We handle all aspects of the usability test, including study design, participant recruitment, facility booking and setup, data collection, root cause analysis, and the HFE/UE report.
Why do I need it?
Demonstrates device can be used by the intended users, under expected use conditions, without serious harm to patient or user. It is required by the FDA during submission for most medical device products, meets requirements for IEC 62366-1.
Are you ready to begin on your product?


