Formative. Summative. Positive.™

Engineering medical products that make sense to the people who use them.
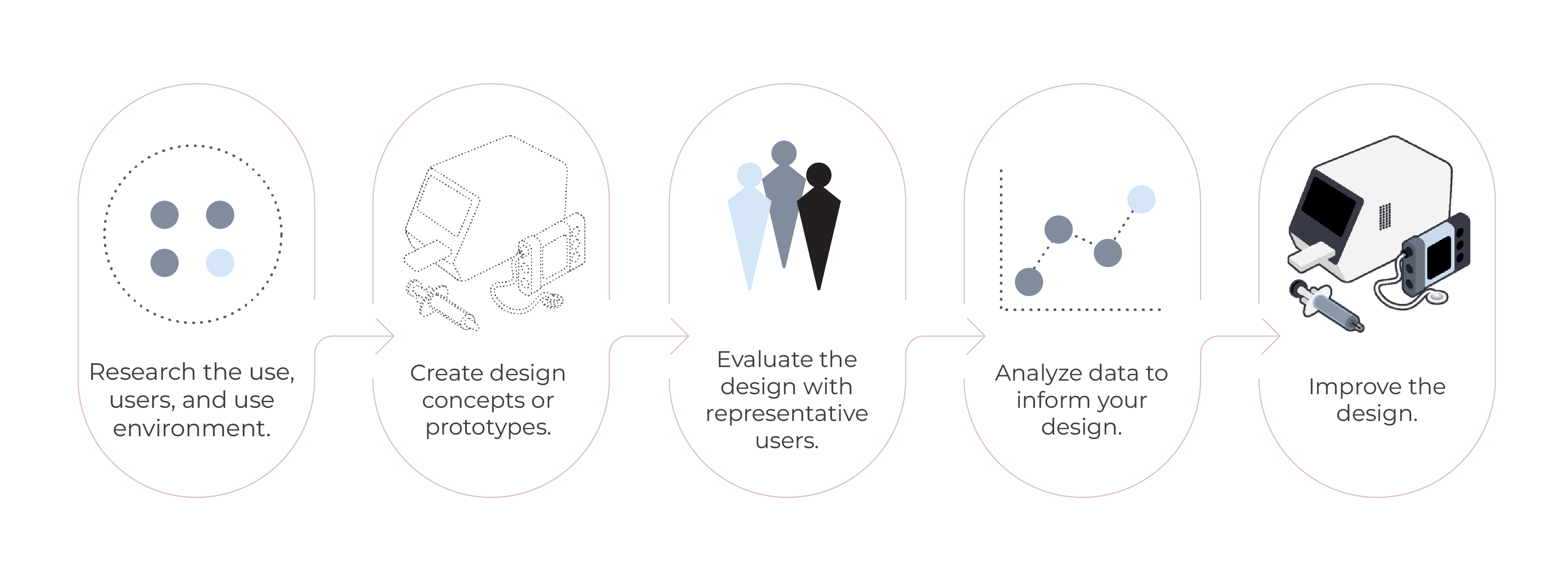
Design research service is all about understanding the right problem to solve for the right user. We use a mix of qualitative and quantitative methods—to gather valuable insights.
Build empirical evidence of your product’s safety, effectiveness, and user experience. This service aims to explore the product’s UI design strengths, weaknesses, and unanticipated use errors with intended users.
Various methods are available depending to your product’s needs, including but not limited to usability testing, expert reviews, and cognitive walkthroughs.
Satisfies IEC-62366-1 and FDA Guidance for HFE/UE.
Comprehensive Summative Human Factors Validation Testing to provide evidence for safe and effective use for submission to the FDA and EU.
We handle all aspects of the usability test, including study design, participant recruitment, facility booking and setup, data collection, root cause analysis, and the HFE/UE report.
Satisfies IEC-62366-1 and FDA Guidance(s) for HFE/UE.
Helps identify the nature of the use errors that users could make and inform your risk mitigation strategies. Method also helps establish usability requirements.
Use-Related Risk Analysis documents potential hazards and harms intended users may encounter via interaction with the product’s user interface. Analysis may also include review of customer complaints, reports or post market surveillance data or other similar product data. Must have for summative testing.
Provides an array of services for Instructions for Use (IFU) and technical writing. Including visual design, layout, and evidence based approach.
This method is for identifying usability problems based on established usability engineering and human factors principles. It is a cost effective and quick way to evaluate your user interface to identify design issues at any point during the design process.
A specialized approach to understanding and interpreting human behavior within specific cultural contexts. This service involves immersive field studies, interviews, and observations to provide businesses with valuable insights into user experiences, preferences, and cultural nuances, enabling them to tailor products and services effectively.
We can evaluate your product’s current design and documentation, and identify if any missing components to meet FDA and 62366-1, along with a roadmap to help complete any requirements.
Your Usability Engineering File (UEF) is the best way to organize your human factors design effort and documents evidence of the human factors process.
Our process is designed to help you deliver a quality, human-centered product.
We have had the pleasure of working with clients from across the medtech industry.









WHAT OUR CLIENTS SAY
"HDFC consistently met all agreed-upon deliverables and timelines, ensuring a smooth workflow throughout the project. Their proactive communication and expertise made them a valuable partner. Despite the many providers in this field, this team's genuine care and enjoyment for their work added a fun and engaging dimension to our collaboration.
We highly recommend their services to any organization looking to enhance their product design and user experience."
Patricio Keegan, Head of Global Marketing
"HF Design Consulting has been a trusted partner. Their medical device HF expertise runs deep, across different products, in addition to the innate strengths to brainstorm and problem-solve while recognizing constraints. It’s great to have such a qualified, local company as a go-to for HF research support. They are always a pleasure to work with."
NANCY TERJUNG, DIRECTOR OF HUMAN FACTORS RESEARCH
"As a repeat customer of HDFC, I am beyond pleased with the level of experience, the commitment to customers, and ability to tailor the Human Factors testing to fit my company's needs.
As a former VP of Product Development for a Biotechnology company in San Diego where I was first introduced to HF Design Consulting, and now where I manage multiple projects for different companies, my first choice for Human Factors Engineering and Usability Engineering is HF Design Consulting."
John Rodriguez, President PPDI



